Answer:
The yield percent is 77.68%
Step-by-step explanation:

1 mole of
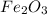 yields 2 moles of Fe.
yields 2 moles of Fe.
So, 11.2 moles of Iron(III) oxide must yield 22.4 moles of Fe theoretically. This is according to the balanced equation.
Theoretical Yield = 22.4 moles of Fe
Experimental Yield = 17.4 moles of Fe
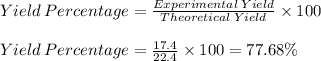
Therefore, the yield percent is 77.68%