Answer:
1.72 days
Explanation:
In 8 days the radioactivity of 1 mg of a radioactive element decreases to 4%.
Initial weight of radioactivity is 1 mg
It is decreases by 4%
4% of 1mg = 0.04 mg
A= ab^{t}
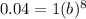
take 8th root on both sides
b=0.6687
The equation becomes
A= 1(0.6687)^{t}
To find half life of the element we replace A with 0.5
0.5= 1(0.6687)^{t}
take ln on both sides

Divide both sides by ln(0.6687)
so t= 1.72244
t= 1.72 days