Answer:
There are 6.024 grams of sodium hydroxide in the solution.
Step-by-step explanation:
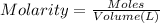
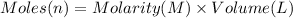
Moles of sodium hydroxide = n
Volume of sodium hydroxide solution = 251.0 mL = 0.251 L
Molarity of the sodium hydroxide = 0.600 M
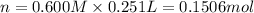
Mass of 0.1506 moles of NaOH :
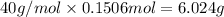
There are 6.024 grams of sodium hydroxide in the solution.