Answer:
Age of mummy when 5 g carbon-14 remains = 6000 years
nitrogen-14 produced during this period = 5 g
Mass of Carbon 14 in another 6000 years = 2.5 g
Amount of Nitrogen-14 in 12 thousand years = 7.5 g
Step-by-step explanation:
Carbon dating is a method used for age determination by analyzing the decay
of carbon-14 to nitrogen-14
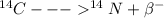
one neutron in carbon-14 splits into one proton and electron
half life (time to decay half of the total mass) of carbon-14 is 5730. here we can approximate this as 6000 years.
initially there is 10 g carbon-14 now remains 5 g that means 1 half-life has passed so,
Age of mummy when 5 g carbon-14 remains = 6000 years
the decayed C-14 is converted to N-14 ( mass of electrons or β particles are negligible) so,
nitrogen-14 produced during this period = (10 - 5 = 5 g
in another 6000 year one more half life will pass the mass of C-14 will further halve ,
Mass of Carbon 14 in another 6000 years = 5 / 2 = 2.5 g
so, Nitrogen remains after 12000 years = 10 - 2.5 = 7.5 g