Answer:
8.533 Mev/nucleon
Step-by-step explanation:
The given elements is:-
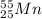
Atomic number : It is defined as the number of electrons or number of protons present in a neutral atom.
Thus, the number of protons = 25
Mass number is the number of the entities present in the nucleus which is the equal to the sum of the number of protons and electrons.
Mass number = Number of protons + Number of neutrons
55 = 25 + Number of neutrons
Number of neutrons = 30
Mass of neutron = 1.008665 amu
Mass of proton = 1.007825 amu
Calculated mass = Number of protons*Mass of proton + Number of neutrons*Mass of neutron
Thus,
Calculated mass = (25*1.007277 + 30*1.008665) amu = 55.441875 amu
Actual mass = 54.9380451 amu
Mass defect = Δm = |54.9380451 - 55.441875| amu = 0.5038299 amu
The conversion of amu to MeV is shown below as:-
1 amu = 931.5 MeV
So, Energy = 0.5038299*931.5 MeV= 469.317552 MeV
Total number of nucleons in the atoms = 55
So, Energy = 469.317552/55 MeV/nucleon = 8.533 Mev/nucleon