Answer:
Hence, the final temperature is 28.3 °C .
Step-by-step explanation:
Given, the edge length of the silver cube = 2.36 cm
The volume of the silver cube =
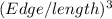 =
=
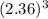 cm³ = 13.144256 cm³
cm³ = 13.144256 cm³
Given, the edge length of the gold cube = 2.67 cm
The volume of the gold cube =
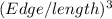 =
=
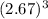 cm³ = 19.034163 cm³
cm³ = 19.034163 cm³
Density is defined as:-
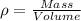
or,
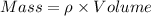
So, Density of silver = 10.5 g/cm³
Thus, Mass of the silver cube = 10.5 g/cm³ * 13.144256 cm³ = 138.0147 g
So, Density of gold = 19.3 g/cm³
Thus, Mass of the gold cube = 19.3 g/cm³ * 13.144256 cm³ = 253.6841 g
So, Density of water = 1 g/cm³
Given, Volume = 108.0 mL = 108.0 cm³
Thus, Mass of the water = 1 g/cm³ * 108.0 cm³ = 108.0 g
Heat gain by water = Heat lost by gold + Heat lost by silver
Thus,
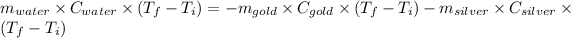
Where, negative sign signifies heat loss
Or,
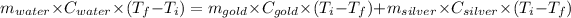
For water:
Mass = 108.0 g
Initial temperature = 19.7 °C
Specific heat of water = 4.184 J/g°C
For gold:
Mass = 253.6841 g
Initial temperature = 87.9 °C
Specific heat of water = 0.1256 J/g°C
For silver:
Mass = 138.0147 g
Initial temperature = 87.9 °C
Specific heat of water = 0.2386 J/g°C
So,
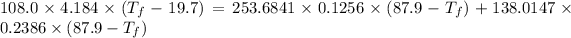
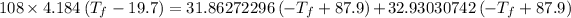
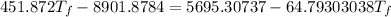
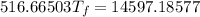
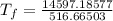
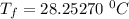
Hence, the final temperature is 28.3 °C .