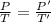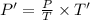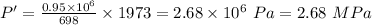Answer:
the pressure at the end of the combustion is 2.68 MPa
Solution:
As per the question:
Initial Pressure, P = 0.95\ MPa
Temperature before combustion,
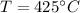 = 273 + 425 = 698 K
= 273 + 425 = 698 K
Temperature after combustion,
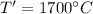 = 1973 K
= 1973 K
Now,
To calculate the pressure at the end of combustion, P':
By using the Pressure-Temperature relation from Gay- Lussac's law: