Answer:
83.72 K
Step-by-step explanation:
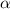 = Polarizability of argon =
= Polarizability of argon =
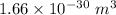
I = First ionization = 1521 kJ/mol
r = Distance between atoms = 3.8 A
R = Gas constant = 8.314 J/mol K
T = Boiling point
Potential energy due to dispersion of gas is given by
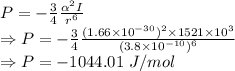
Kinetic energy is given by
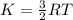
The potential and kinetic energy will balance each other
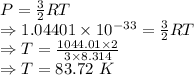
The boiling point of argon is 83.72 K