Answer:
The molar mass of
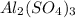 is 342.145 g/mol
is 342.145 g/mol
Step-by-step explanation:
The molar mass is stated as the “mass per unit amount of substance”of a given chemical compound
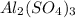 is Aluminium sulphate. They easily dissolve in water. It is primarily used as a 'coagulating agent' in the drinking water purification and also in waste and sewage water treatment plants,
is Aluminium sulphate. They easily dissolve in water. It is primarily used as a 'coagulating agent' in the drinking water purification and also in waste and sewage water treatment plants,
We know,
Atomic weight of Aluminium = 26.981
Atomic weight of Sulphur= 32.065
Atomic weight of Oxygen = 15.9994
Now molar mass of
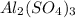 (Aluminium sulphate) is
(Aluminium sulphate) is
=>2(26.981)+3(32.065+4(15.999))
=>53.962 +3(32.065+63.996)
=>53.962+3(96.061)
=>53.962+288.183
=>342.145