Answer:
17.934 kg of water
Step-by-step explanation:
If balanced equation is not given; this format can come in handy.
For any alkane of the type : CₙH₂ₙ₊₂ , it's combustion reaction will follow:
2CₙH₂ₙ₊₂ + (3n+1) O₂ → (2n)CO₂ + 2(n+1) H₂O
For butane:
2C₄H₁₀(g) + 13O₂(g) → 8CO₂(g) + 10H₂O(l)
2 moles of butane gives 10 moles of water.
1 mol of any substance has Avogadro number(N) of molecules in it( 6.022 x 10²³)
Mass of 1 mole of any substance is equal to it's molar mass
So, if 2 x N molecules of butane gives 10 x 18 g of water.
Then 1.2 x 10²⁶ molecules will give:
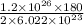
= 17.934 x 10³ g of water
= 17.934 kg of water