Answer:
11.0845g of
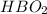 is produced from the combustion of 96.9 g of
is produced from the combustion of 96.9 g of
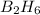
Step-by-step explanation:
Given:
mass of
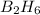 used in combustion= 96.9 g
used in combustion= 96.9 g
To find:
amount of
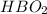 produced=?
produced=?
Solution:
Let us find the molecular mass of
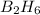
Atomic weight of Boron(B)=10.811
Atomic weight of Hydrogen(H)=1.007
Now the molecular mass of
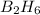
=>2(10.811)+6(1.007)
=>21.622 x 6.042
=>27.664
In the given 96.9 g of
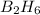 , the number of moles of
, the number of moles of
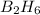 present is
present is
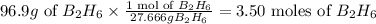
So in 96.9 g of B2H6, 3.50 moles of B2H6 is present which is converted into 2 molecules of
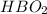
The molecular mass of
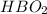 is
is
Atomic weight of Boron(B)=10.811
Atomic weight of Hydrogen(H)=1.007
Atomic weight of Oxygen(O)=15.9994
=> 10.811+1.007+2(15.9994)
=>10.811+1.007+31.998
=>43.816
Convert 3.50 moles of
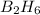 to HBO2
to HBO2
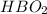
=>
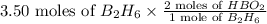
=>
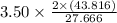
=>
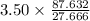
=>
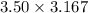
=>11.0845