Answer:
α = -0,885°
Step-by-step explanation:
The specific rotation [α] is defined as:
[α] = α/ c×l (1)
Where α is the observed rotation, c is concentration in g/mL, and l is optical path length in dm.
A racemic mixture has no optical activity. As the solution of (R)-2-butanol is mixed with an equal volume of the racemic mixture the concentration of the original solution decreases twice. That is 1,72M/2 = 0,86M. This concentration in g/mL is:
0,86mol/L×
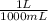 ×
×
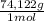 = 0,0637 g/mL
= 0,0637 g/mL
Replacing in (1):
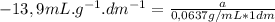
α = -0,885°
I hope it helps!