Answer:
78.89 g of CO is required.
Step-by-step explanation:

Fe = 55.85 g/mol
O = 15.99 g/mol
C = 12.01 g/mol
2 moles of Fe is produced on reacting 1 mole of
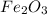 .
.
2 moles of Fe is produced on reacting 3 moles of CO.
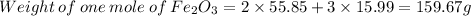
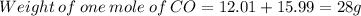
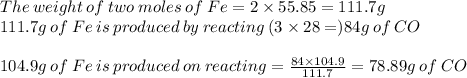
Hence, 78.89 g of CO on reacting with excess of ferric oxide will produce 104.9 g of Fe.