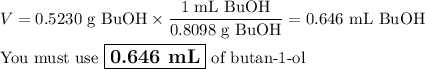Answer:

Step-by-step explanation:
We will need a balanced chemical equation with masses and molar masses, so, let's gather all the information in one place.
MM: 74.12 184.24
CH₃CH₂CH₂CH₂OH + (C₆H₅)₂CHOH ⟶ Product
m/g: 1.30
ρ/g·mL⁻¹: 0.8098
1. Moles of benzhydrol (BH)
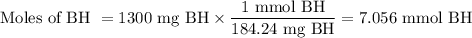
2. Moles of butan-1-ol (BuOH)
The molar ratio is 1 mmol BuOH:1 mmol BH.
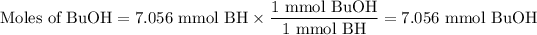
3. Mass of BuOH
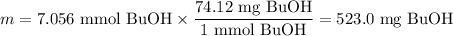
4. Volume of BuOH