Answer:
a) 1.39 g ; b) O₂ is limiting reactant, NH₃ is excess reactant; c) 0.7 g
Step-by-step explanation:
We have the masses of two reactants, so this is a limiting reactant problem.
We will need a balanced equation with masses, moles, and molar masses of the compounds involved.
1. Gather all the information in one place with molar masses above the formulas and masses below them.
MM: 17.03 32.00 30.01
4NH₃ + 5O₂ ⟶ 4NO + 6H₂O
Mass/g: 1.5 1.85
2. Calculate the moles of each reactant
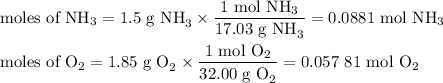
3. Calculate the moles of NO we can obtain from each reactant
From NH₃:
The molar ratio is 4 mol NO:4 mol NH₃

From O₂:
The molar ratio is 4 mol NO:5 mol O₂
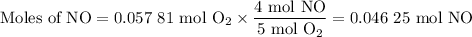
4. Identify the limiting and excess reactants
The limiting reactant is O₂ because it gives the smaller amount of NO.
The excess reactant is NH₃.
5. Calculate the mass of NO formed
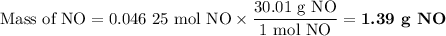
6. Calculate the moles of NH₃ reacted
The molar ratio is 4 mol NH₃:5 mol O₂
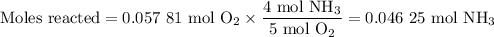
7. Calculate the mass of NH₃ reacted
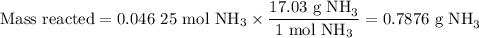
8. Calculate the mass of NH₃ remaining
Mass remaining = original mass – mass reacted = (1.5 - 0.7876) g = 0.7 g NH₃