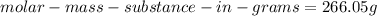Answer: 266.055 g
Step-by-step explanation:
We have the following data related to this unknown substance:
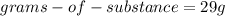 are the grams of substance
are the grams of substance
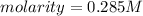 is the molarity of the substance, given by the following formula:
is the molarity of the substance, given by the following formula:
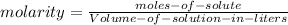 (1)
(1)
In this case the volume of solution is
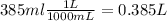
Finding the moles of solute:
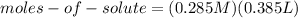 (2)
(2)
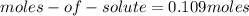 (3)
(3)
Now we have to convert this to grams by the following relation:
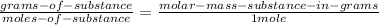 (4)
(4)
Finding
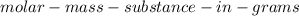 :
:
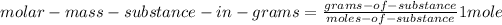 (5)
(5)
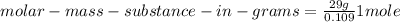 (6)
(6)