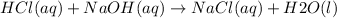"Double-displacement reactions" are the "positively" charged parts of "two compounds" trading places.
Option: D
Step-by-step explanation:
"Double displacement reaction" is one of the type of chemical reactions. It is a process in which one component each of both the reacting molecules get exchanged to form the products which is precipitate obtained from mother liquor while rest is filtrate. Following is the reaction:
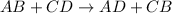
In double displacement reaction two compounds react, then positive ions (cation) switch their places, forming "two new compounds" or "products". For example: