Answer:
Percentage of silver and sulphur in the compound is 87.08% and 12.91% respectively
Step-by-step explanation:
Given:
Amount of silver = 29.0 g
Amount of sulphur= 4.30 g
To find:
Percent composition of the compound=?
Solution:
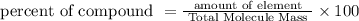
Total Molecule mass = amount of silver + amount of sulphur
Total Molecule mass = 29+4.3
Total Molecule mass = 33.3g
Percentage of silver:
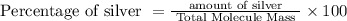
=>

=>
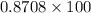
=> 87.08%
Percentage of silver:
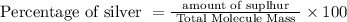
=>

=>
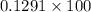
=> 12.91%