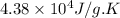Answer : The entropy change for the surroundings when 1.74 moles of Na(s) react at standard conditions is
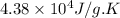
Explanation :
The given balanced chemical reaction is,
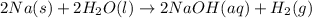
First we have to calculate the enthalpy of reaction
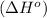 .
.
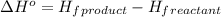
![\Delta H^o=[n_(NaOH)* \Delta H_f^0_((NaOH))+n_(H_2)* \Delta H_f^0_((H_2))]-[n_(Na)* \Delta H_f^0_{(Na)+n_(H_2O)* \Delta H_f^0_((H_2O))]](https://img.qammunity.org/2020/formulas/chemistry/college/d5ihpzml49bq3ua6u11sh2bosjktsnmxnm.png)
where,
We are given:
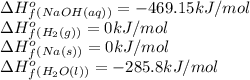
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(2* -469.15)+(1* 0)]-[(2* 0)+(2* -285.8)]=-652.5kJ](https://img.qammunity.org/2020/formulas/chemistry/college/7odv1qpk0v0kh525gw5lmz98f1fr5c8it1.png)
Now we have to calculate the entropy change for surrounding
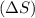 .
.
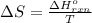
where,
 = change in entropy
= change in entropy
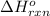 = change in enthalpy = -652.5 kJ
= change in enthalpy = -652.5 kJ
T = temperature = 298 K
Now put all the given values in the above formula, we get:
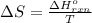
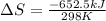
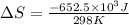
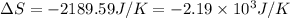
Now we have to calculate the entropy change for the surroundings when 1.74 moles of Na(s) react at standard conditions.
As, 2 moles of Na(s) has entropy change =
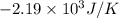
And, 1.74 moles of Na(s) has entropy change =
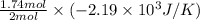
Thus, 23 g of Na(s) has entropy change =
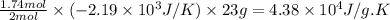
Therefore, the entropy change for the surroundings when 1.74 moles of Na(s) react at standard conditions is