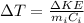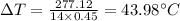Answer:
The rise in temperature is
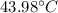
Solution:
As per the question:
Mass of hammer, M = 1.30 kg
Speed of hammer, v = 7.3 m/s
Mass of iron,
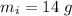
No. of blows, n = 8
Specific heat of iron,
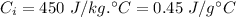
Now,
To calculate the temperature rise:
Transfer of energy in a blow = Change in the Kinetic energy
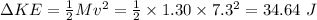
For 8 such blows:
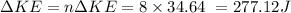
Now, we know that: