Step-by-step explanation:
Let us assume that total mass of the solution is 100 g. And, as it is given that acetic acid solution is 12% by mass which means that mass of acetic acid is 12 g and 88 g is the water.
Now, calculate the number of moles of acetic acid as its molar mass is 60 g/mol.
No. of moles =
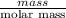
=
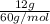
= 0.2 mol
Molarity of acetic acid is calculated as follows.
Density =
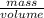
1 g/ml =
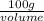
volume = 100 ml
Hence, molarity =
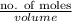
=
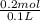
= 2 mol/l
As reaction equation for the given reaction is as follows.

So, moles of NaOH = moles of acetic acid
Let us suppose that moles of NaOH are "x".
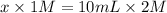 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)
x = 20 L
Thus, we can conclude that volume of NaOH required is 20 ml.