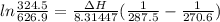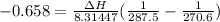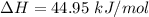Answer:
Enthalpy is 44.95 kJ/mol
Solution:
As per the question:
Temperature, T = 270.6 K
Temperature, T' = 287.5 K
Pressure, P = 324.5 mmHg
Pressure, P' = 626.9 mmHg
Now,
To calculate the enthalpy, we make use of the Clausius-Clapeyron eqn:
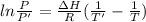
where
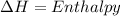
R = Rydberg's constant
Substituting suitable values in the above eqn: