Answer:
A.The dipole moment is a scalar quantity.
Step-by-step explanation:
We know that
Dipole moment :It measure the polarity of chemical bond in a molecule .
The electric dipole moment is equal to product of any charge (positive or negative ) and the distance between the two charges.
Mathematical representation:
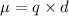
Where
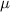 =Dipole moment
=Dipole moment
q=Charge on atom or particle
d=Distance between two charged particles
It helps to find out the molecule is polar or non- polar.
When the dipole moment is zero then the molecule is non-polar.
Dipole moment is a vector quantity.
The direction of dipole moment from negative charge to positive.
When unit of charge is C and unit of distance is m.
Then, unit of dipole moment=C-m
Hence, option A is false.