Answer:
2KMnO₄ + 5H₂C₂O₄ + 3H₂SO₄ → 2MnSO₄ + 10CO₂ + 8H₂O + K₂SO₄
0,02658g of La in the unknown
Step-by-step explanation:
The reaction of La₂(C₂O₄)₃ with acid is:
La₂(C₂O₄)₃ + 6H⁺ → 3H₂C₂O₄ + 2La³⁺
The titration of H₂C₂O₄ with KMnO₄ is:
2KMnO₄ + 5H₂C₂O₄ + 3H₂SO₄ → 2MnSO₄ + 10CO₂ + 8H₂O + K₂SO₄
The moles of KMnO₄ that react are:
0,006363M KMnO₄×0,01804L = 1,148x10⁻⁴moles of KMnO₄
By the titration reaction, 2 moles of KMnO₄ react with 5 moles of H₂C₂O₄, that means:
1,148x10⁻⁴moles of KMnO₄×
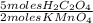 = 2,870x10⁻⁴ moles of H₂C₂O₄.
= 2,870x10⁻⁴ moles of H₂C₂O₄.
In the reaction of La₂(C₂O₄)₃ with acid, 3 moles of H₂C₂O₄ were produced while 2 moles of La³⁺ were produced, that means:
2,870x10⁻⁴ moles H₂C₂O₄×
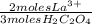 = 1,913x10⁻⁴ moles of La³⁺, in grams -Using molar mass of lanthanum-:
= 1,913x10⁻⁴ moles of La³⁺, in grams -Using molar mass of lanthanum-:
1,913x10⁻⁴ moles of La³⁺×
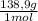 = 0,02658g of La
= 0,02658g of La
There are 0,02658g of La in the unknown
I hope it helps!