Answer:
S= 1.40x10⁻⁵mol/L
Step-by-step explanation:
The Henry's Law is given by the next expression:
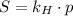 (1)
(1)
where S: is the solubility or concentration of Ar in water,
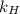 : is Henry's law constant and p: is the pressure of the Ar
: is Henry's law constant and p: is the pressure of the Ar
Since the argon is 0.93%, we need to multiply the equation (1) by this percent:
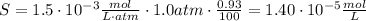
Therefore, the argon solubility in water is 1.40x10⁻⁵mol/L.
Have a nice day!