Answer:
Empirical formula = S₁Cl₂ = SCl₂
Step-by-step explanation:
The empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound.
This compound contains 31.1% sulfur and 68.9% chlorine.
That is 100g of the compound contains 31.1 g of sulfur and 68.9 g of chlorine.
Convert the mass of each element to moles using the molar mass from the periodic table.
Moles of Sulfur =
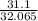
= 0.9699
Moles of Chlorine=
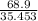
= 1.9434
Divide each mole value by the smallest number of moles calculated.
Units of sulfur =
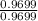
= 1
Units of Chlorine =
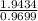
= 2
Empirical formula = S₁Cl₂ = SCl₂