Answer:
2.04 is the pOH of a 0.0092 M CsOH solution.
Step-by-step explanation:
Molarity of cesium hydroxde = [CsOH]= 0.0092
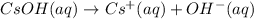
1 mole of cesium hydroxide gives 1 mole of cesium ion and 1 mole of hydroxide ion.
Then 0.0092 M cesium hydroxide will give :
![[OH^-]=1* [CsOH]=1* 0.0092 M=0.0092M](https://img.qammunity.org/2020/formulas/chemistry/college/ugbk1rs0ki3fhx8a53957t92h6moaeidwy.png)
The pOH of the solution is the negative logarithm of concentration of hydroxide ions in a solution.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
![pOH=-\log [0.0092 M]=2.0362\approx 2.04](https://img.qammunity.org/2020/formulas/chemistry/college/3ss38o3annqbbk46ud6nt5b75lnhd93gq1.png)
2.04 is the pOH of a 0.0092 M CsOH solution.