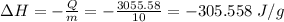Answer:
The enthalpy for dissolution is - 305.558 J/g
Solution:
Mass of the ionic compound, m = 10.00 g
Mass of water, m' = 75.0 g
Initial temperature, T =
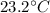
Final Temperature, T' =
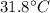
Now,
To calculate the change in enthalpy:
We know that the specific heat of water is 4.18
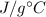
Total mass of the solution, M = m + m' = 10.00 + 75.0 = 85.0 g
Temperature, difference,
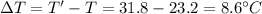
Thus
The heat absorbed by the solution is given by:
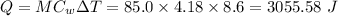
Enthalpy,