Answer:
The expression will be given as:
![[CH_3OH]=K_c* [CO]* [H_2]^2](https://img.qammunity.org/2020/formulas/chemistry/college/ojqfg3c6p8yn61whghheg2mu8s89s5t441.png)
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
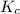
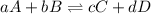
![K_(c)=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2020/formulas/chemistry/high-school/13aayk47tkvc79ferxww1rmrj9bfzrdqu4.png)
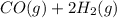 ⇌
⇌
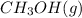
The equilibrium-constant expression for the given reaction is given by:
![K_(c)=([CH_3OH])/([CO][H_2]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/i12thwgyrjhjd3xyjn7t8o7jrf07ldc9wv.png)
If we are given with equilibrium constant and equilibrium concentration of carbon monoxide and hydrogen gas we can determine the concentration of methanol at equilibrium.
The expression will be given as:
![[CH_3OH]=K_c* [CO]* [H_2]^2](https://img.qammunity.org/2020/formulas/chemistry/college/ojqfg3c6p8yn61whghheg2mu8s89s5t441.png)