Answer:
The correct answer is option E.
Step-by-step explanation:
The Gibbs free energy is given by expression:
ΔG = ΔH - TΔS
ΔH = Enthalpy change of the reaction
T = Temperature of the reaction
ΔS = Entropy change
We have :
ΔH = -720.5 kJ/mol = -720500 J/mol (1 kJ = 1000 J)
ΔS = -263.7 J/K
T = 141.0°C = 414.15 K
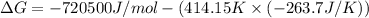
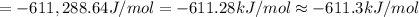
The Gibb's free energy of the given reaction at 141.0°C is -611.3 kJ/mol.