Answer:
6.074 X 10¹⁸ molecules.
Step-by-step explanation:
Molecular mass(MM) of this compound C₂₇H₄₆O
= 27*(MM of C) + 46*(MM of H) + (MM of O)
= 27*(12.0107) + 46* (1.00784) + (15.999)
= 324.2889 + 46.36064 + 15.999
=386.64854 g
The mass of a mole of any compound is called it's molar mass. 1 molar mass has 6.022 X 10²³, or Avogadro's number, C₂₇H₄₆O molecules.
If 386.64854 g of C₂₇H₄₆O has 6.022 X 10²³ molecules of cholesterol, then
3.9mg or 0.0039g of C₂₇H₄₆O will have how many molecules?..
Using unitary method:
Number of molecules =
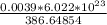
= 6.074 X 10¹⁸ molecules.