Answer:
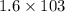 g of K₂SO₄ will be produced
g of K₂SO₄ will be produced
Step-by-step explanation:
Given
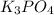 is available in excess
is available in excess
Reaction:
Cr₂(SO₄)₃ + 2K₃PO₄ ----------> 3K₂SO₄ + 2CrPO₄
From the above reaction, it is clear that the moles of (Cr₂(SO₄)₃),
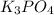 and K₂SO₄ are 1, 2 and 3 respectively
and K₂SO₄ are 1, 2 and 3 respectively
We can say that 1 mole of chromium(iii) sulfate (Cr₂(SO₄)₃) react with 2 mole of potassium phosphate (K₃PO₄) to produce 3 mole of K₂SO₄
molar mass of Cr₂(SO₄)₃ = 147 g/mol
molar mass of K₃PO₄ = 212 g/mol
molar mass of K₂SO₄ = 174 g/mol
We can write as;
Cr₂(SO₄)₃ + 2K₃PO₄ ----------> 3K₂SO₄ + 2CrPO₄
1 mol (147 g/mol) 2 mol (212 g/mol) 3 mol (174g/mol)
Therefore, we have
Cr₂(SO₄)₃ + 2K₃PO₄ ----------> 3K₂SO₄ + 2CrPO₄
147 g 424 g 522 g
So, we can see that 147 g of Cr₂(SO₄)₃ reacts with 424 g of 2K₃PO₄ to produce 522 g of K₂SO₄
147 g of Cr₂(SO₄)₃ = 522 g of K₂SO₄
So, 450 g of Cr₂(SO₄)₃ =
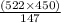 g of K₂SO₄ = 1597.959 g =
g of K₂SO₄ = 1597.959 g =
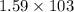 g =
g =
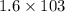 g
g
So,
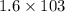 g of K₂SO₄ will be produced
g of K₂SO₄ will be produced