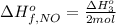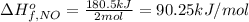Answer:
The standard molar enthalpy of formation of 1 mole of NO gas is 90.25 kJ/mol.
Step-by-step explanation:
We have :
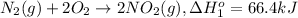
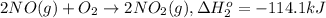
To calculate the standard molar enthalpy of formation
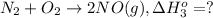 ...[3]
...[3]
[1] - [2] = [3] (Hess's law)
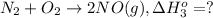
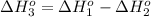
![\Delta H^o_(3)=66.4 kJ - [ -114.1 kJ] = 180.5 kJ](https://img.qammunity.org/2020/formulas/chemistry/college/yozoxg7uhmp3y1a69ml9amc0h9rfbvbkqw.png)
According to reaction [3], 1 mole of nitrogen gas and 1 mole of oxygen gas gives 2 mole of nitrogen monoxide.
So, the standard molar enthalpy of formation of 1 mole of NO gas :