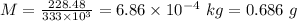Answer:
0.686 g of ice melts each second.
Solution:
As per the question:
Cross-sectional Area of the Copper Rod, A =
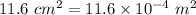
Length of the rod, L = 19.6 cm = 0.196 m
Thermal conductivity of Copper, K =
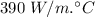
Conduction of heat from the rod per second is given by:
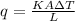
where
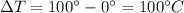 = temperature difference between the two ends of the rod.
= temperature difference between the two ends of the rod.
Thus
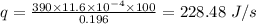
Now,
To calculate the mass, M of the ice melted per sec:
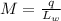
where
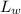 = Latent heat of fusion of water = 333 kJ/kg
= Latent heat of fusion of water = 333 kJ/kg