Answer:
599.26 grams of potassium sulfate will be produced.
Step-by-step explanation:
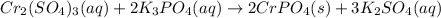
Moles of chromium (III) sulfate =
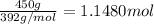
According to reaction, 1 mole of chromium (III) sulfate gives 3 moles of potassium sulfate.
Then 1.1480 moles of chromium (III) sulfate will give:
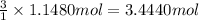
Mass of 3.4440 moles of potassium sulfate:
= 3.4440 mol × 174 g/mol = 599.26 g
599.26 grams of potassium sulfate will be produced.