Answer: The mass of nickel that was originally heated is 28.4 grams.
Step-by-step explanation:
When metal is dipped in water, the amount of heat released by metal will be equal to the amount of heat absorbed by water.
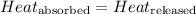
The equation used to calculate heat released or absorbed follows:
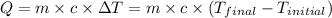
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2020/formulas/chemistry/college/bm2kxludvecqgwsul6e5upktie71evfnq2.png) ......(1)
......(1)
where,
q = heat absorbed or released
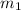 = mass of nickel = ?
= mass of nickel = ?
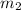 = mass of water = 150.0 g
= mass of water = 150.0 g
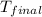 = final temperature = 25.0°C
= final temperature = 25.0°C
 = initial temperature of nickel = 99.8°C
= initial temperature of nickel = 99.8°C
 = initial temperature of water = 23.5°C
= initial temperature of water = 23.5°C
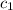 = specific heat of nickel = 0.444 J/g°C
= specific heat of nickel = 0.444 J/g°C
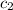 = specific heat of water= 4.186 J/g°C
= specific heat of water= 4.186 J/g°C
Putting values in equation 1, we get:
![m_1* 0.444* (25.0-99.8)=-[150.0* 4.186* (25.0-23.5)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/qbp2sem6mozmih9tbx0x0l4st6fkhginju.png)
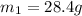
Hence, the mass of nickel that was originally heated is 28.4 grams.