Answer:
Pb-210 that lead-210 is the product of second step.
Step-by-step explanation:
Alpha decay : When a larger nuclei decays into smaller nuclei by releasing alpha particle. In this process, the mass number and atomic number is reduced by 4 and 2 units respectively.
General representation of alpha decay :
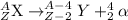
Beta decay : In this process, a neutron is converted into a proton and an electron. In this decay, the atomic number is increased by 1 unit.
General representation of beta decay :
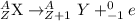
Step 1. Bi undergoes α decay;
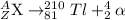
81 = Z-2
Z = 83
210 = A - 4
A = 214
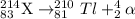
Step 2: Tl-210 undergoes β decay:
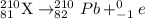
Pb-210 that lead-210 is the product of second step.