Answer:
Step-by-step explanation:
Electron's kinetic energy = 2 eV
= 2 x 1.6 x 10⁻¹⁹ J
1/2 m v² = 3.2 x 10⁻¹⁹
1/2 x 9.1 x 10⁻³¹ x v² = 3.2 x 10⁻¹⁹
v² = .703 x 10¹²
v = .8385 x 10⁶ m/s
Electrons revolve in a circular orbit when forced to travel in a magnetic field whose radius can be expressed as follows
r = mv / Bq
where m , v and q are mass , velocity and charge of electron .
here given magnetic field B = 90 mT
= 90 x 10⁻³ T
Putting these values in the expression above
r = mv / Bq
=
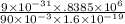
= .052 mm.