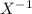Answer: They ionize by the gain of one electron
Step-by-step explanation:
Group 7 elements have seven electrons on their outermost shell, in order to have a complete octet structure, the go into bonding with other elements by gaining one more electron to have eight complete outermost shell electron (valence electron). They thereby ionize by gain one electron forming X