Answer:
The correct answer is option A.
Step-by-step explanation:
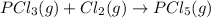
Enthalpy of formation of
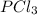 gas =
gas =
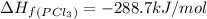
Enthalpy of formation of chlorine gas =
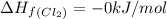
Enthalpy of formation of
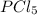 gas =
gas =
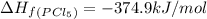
The equation used to calculate enthalpy change is of a reaction is:
![\Delta H_(rxn)=\sum [n* \Delta H_f(product)]-\sum [n* \Delta H_f(reactant)]](https://img.qammunity.org/2020/formulas/chemistry/college/qr2q81u7h1zshv5jyx27y3fot8dv8ajdt7.png)
For the given chemical reaction:
![\Delta H_(rxn)=[(1* \Delta H_f_((PCl_5)))]-[(1* \Delta H_f_((PCl_3)))+(1* \Delta H_f_((Cl_2)))]](https://img.qammunity.org/2020/formulas/chemistry/college/70zzje4doprfdhjag4fvdiyrv5nd32x81e.png)
![=[1* (-374.9 kJ/mol)]-[1* (-288.7 kJ/mol)+1* 0 kJ/mol]](https://img.qammunity.org/2020/formulas/chemistry/college/9piehmsz9paqjlfn8ursas4f4ju4dy11e2.png)
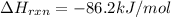
Entropy of of
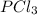 gas =
gas =
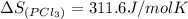
Entropy of chlorine gas =
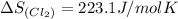
Entropy of
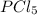 gas =
gas =
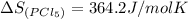
The equation used to calculate enthalpy change is of a reaction is:
The equation used to calculate enthalpy change is of a reaction is:
![\Delta S_(rxn)=\sum [n* \Delta S_(product)]-\sum [n* \Delta S_(reactant)]](https://img.qammunity.org/2020/formulas/chemistry/college/n9froxvk4a758j758zsm1eotjk1gfbg9me.png)
The equation for the entropy change of the above reaction is:
![\Delta S_(rxn)=[(1* \Delta S_((PCl_5)))]-[(1* \Delta S_((PCl_3)))+(1* \Delta S_((Cl_2)))]](https://img.qammunity.org/2020/formulas/chemistry/college/ld0554nlp2huw2jbat0hmjdk5b6ghtznv4.png)
![=[1* 364.2 J/molK]-[1* 311.6 J/mol K+1* 223.1 J/mol K]](https://img.qammunity.org/2020/formulas/chemistry/college/ngot12tj0wt06xblh35utbcrgmfeznkytu.png)
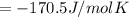
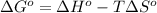
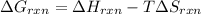
At equilibrium ,
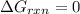
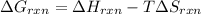

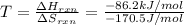
1 kJ = 1000 J
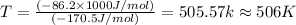
At 506 K reaction will be at an equilibrium.