Answer:
The answer is: 11759 Hz
Step-by-step explanation:
Given: Chemical shift: δ = 211.5 ppm, Spectrometer frequency = 556 MHz = 556 × 10⁶ Hz
In NMR spectroscopy, the chemical shift (δ), expressed in ppm, of a given nucleus is given by the equation:
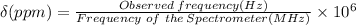
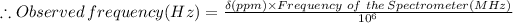
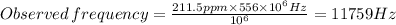
Therefore, the signal is at 11759 Hz from the TMS.