Answer:
The final product of the reaction is (2S,3S)-2-ethoxy-3-methylpentane.
Step-by-step explanation:
The given reaction undergoes
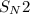 mechanism in which the nucleophile attacks the backside and it is substituted by the elimination of bromine.
mechanism in which the nucleophile attacks the backside and it is substituted by the elimination of bromine.
Due to the backside attack of nucleophile , the inverse in stereo-chemistry is observed.
After the substitution of ethoxy group, the configuration is assigned according to the priority it shows clock wise direction(R) - configuration.
When hydrogen faces the front side , it results shows inverse configuration i.e, S- configuration.
The chemical reaction is as follows.