Answer:
Concentration of dissolved nitrogen = 5.2 × 10⁻⁴ mol/L
Step-by-step explanation:
More the pressure of the gas, more will be its solubility.
So, for two different pressure, the relation between them is shown below as:-
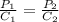
Given ,
P₁ = 1 atm
P₂ = 0.76 atm
C₁ = 6.8 × 10⁻⁴ mol/L
C₂ = ?
Using above equation as:
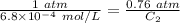
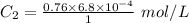
Concentration of dissolved nitrogen = 5.2 × 10⁻⁴ mol/L