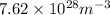Answer : The number of free electrons per cubic meter for silver are
Explanation :
To calculate the number of free electrons per cubic meter for silver by using the following equation:
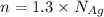
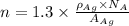
where,
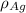 = density of Ag =
= density of Ag =
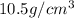
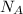 = Avogadro's number =
= Avogadro's number =
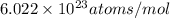
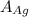 = 107.87 g/mol
= 107.87 g/mol
Now put all the given values in the above formula, we get:
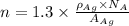
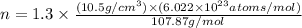
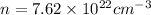

conversion used :
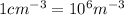
Therefore, the number of free electrons per cubic meter for silver are