Answer:
1.415 gram of the element will be left.
Explanation:
The decay of radioactive element Krypton-91 can be formulated as
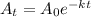 ............ (1)
............ (1)
where,
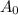 is the initial amount of the element.
is the initial amount of the element.
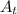 is the amount of element left after t seconds.
is the amount of element left after t seconds.
And k is a rate constant.
Now, given that the half-life of the element is 10 seconds.
So, from equation (1) we get
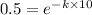
taking ln on both sides, we get.
ln 0.5 = -10k
⇒ k = 0.0693
So, the equation (1) becomes
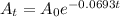 ........ (2)
........ (2)
Now, if 16 gram of the element are initially present, then we asked to determine the amount of the element left after 35 seconds.
So, from equation (2) we have
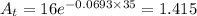 gm.
gm.
So, 1.415 gram of the element will be left. (Answer)