Answer:
The percentage of amine is protonated is 63,07%
Step-by-step explanation:
The reaction of an amine RNH₃ (weak base) with water is:
RNH₃ + H₂O ⇄ RNH₄⁺ + OH⁻
The kb is defined as:
![kb = ([RNH_(4)^+][OH^-])/([NH_(3)])](https://img.qammunity.org/2020/formulas/chemistry/college/iy1ikmjvbe7awu6u18fbsjvihrcc9xwloe.png)
As kb = 4,004x10⁻⁵ and [OH⁻] is
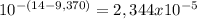 :
:
![4,004x10^(-5) = ([RNH_(4)^+][2,34x10^(-5)])/([NH_(3)])](https://img.qammunity.org/2020/formulas/chemistry/college/nhzs3e15zlhy59hmas3bqkwwyuqlm5pj4q.png)
1,708 = [RNH₄⁺] / [RNH₃] (1)
As the total amine is a 100%:
[RNH₄⁺] + [RNH₃] = 100% (2)
Replacing (1) in (2):
1,708 [RNH₃]+ [RNH₃] = 100%
2,708 [RNH₃] = 100%
[RNH₃] = 36,93%
Thus,
[RNH₄⁺] = 63,07%
The percentage of amine protonated is 63,07%
I hope it helps!