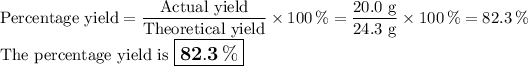Answer:
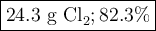
Step-by-step explanation:
We are given the masses of two reactants and asked to determine the mass of the product.
This looks like a limiting reactant problem.
1. Assemble the information
We will need a balanced equation with masses and molar masses, so let’s gather all the information in one place.
MM: 86.94 36.46 70.91
MnO₂ + 4HCl ⟶ MnCl₂ + Cl₂ + 2H₂O
Mass/g: 86.0 50.0 20.00
2. Calculate the moles of each reactant
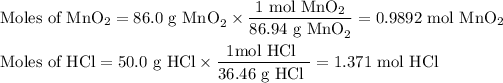
3. Calculate the moles of Cl₂ formed from each reactant
From MnO₂:
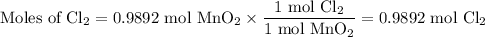
From HCl:
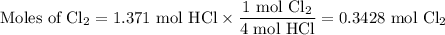
4. Identify the limiting reactant
The limiting reactant is HCl, because it forms fewer moles of Cl₂.
5. Calculate the theoretical yield of Cl₂
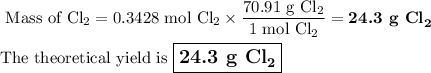
6. Calculate the percentage yield of Cl₂