Answer:
The weight percent in the sample is 17,16%
Step-by-step explanation:
The dissolution of the Ce(IV) salt provides free Ce⁴⁺ that reacts, thus:
2Ce⁴⁺ + 3I⁻ → 2Ce³⁺ + I₃⁻
I₃⁻ + 2S₂O₃²⁻ → 3I⁻ + S₄O₆²⁻
The moles in the end point of S₂O₃⁻ are:
0,01302L×0,03247M Na₂S₂O₃ = 4,228x10⁻⁴ moles of S₂O₃²⁻.
As 2 moles of S₂O₃⁻ react with 1 mole of I₃⁻, the moles of I₃⁻ are:
4,228x10⁻⁴ moles of S₂O₃⁻×
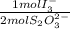 = 2,114x10⁻⁴ moles of I₃⁻
= 2,114x10⁻⁴ moles of I₃⁻
As 2 moles of Ce⁴⁺ produce 1 mole of I₃⁻, the moles of Ce⁴⁺ are:
2,114x10⁻⁴ moles of I₃⁻×
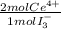 = 4,228x10⁻⁴ moles of Ce(IV).
= 4,228x10⁻⁴ moles of Ce(IV).
These moles are:
4,228x10⁻⁴ moles of Ce(IV)×
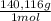 = 0,05924 g of Ce(IV)
= 0,05924 g of Ce(IV)
As was taken an aliquot of 25,00mL from the solution of 250,0mL:
0,05924 g of Ce(IV)×
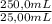 =0,5924g of Ce(IV) in the sample
=0,5924g of Ce(IV) in the sample
As the sample has 3,452g, the weight percent is:
0,5924g of Ce(IV) / 3,452g × 100 = 17,16 wt%
I hope it helps!