Step-by-step explanation:
The given data is as follows.
Thickness = 0.8 cm =
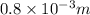 (As 1 m = 1000 cm)
(As 1 m = 1000 cm)
Diameter = 14.0 cm, Conductivity =
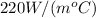
mass = 1.2 g, L =
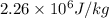
Now, we will calculate the heat of vaporization for water as follows.
Q = mL
Q =
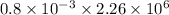
Q = 1808 J
So, rate of heat transfer in 1 sec will be as follows.
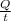 = 1808 J/s
= 1808 J/s
Thus, we can conclude that 1808 J/s heat is transfered to the boiling water in one second.