a. AlCl₃ ⇒ limiting reactant(smaller ratio)
Cu ⇒ excess reactant
b. the mass of leftover reactant : 7.207 g
Further explanation
Given
25 g Cu
25 g AlCl3
Required
a. the excess and limiting reactants
b. the mass of leftover reactant
Solution
Reaction
3Cu + 2AlCl₃ ⇒ 3CuCl₂ + 2Al
mol Cu(Ar = 63.5 g/mol) :
mol = mass : Mw
mol = 25 : 63.5
mol = 0.394
mol AlCl3(MW=133,34 g/mol) :
mol = 25 : 133,34 g/mol
mol = 0.187
mol ratio to reaction coefficient Cu : AlCl₃ =
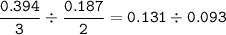
AlCl₃ ⇒ limiting reactant(smaller ratio)
Cu ⇒ excess reactant
b. the mass of leftover reactant :
mol Cu = 3/2 x 0.187 = 0.2805
mol left = 0.394 - 0.2805 = 0.1135
mass = 0.1135 x 63.5 = 7.207 g